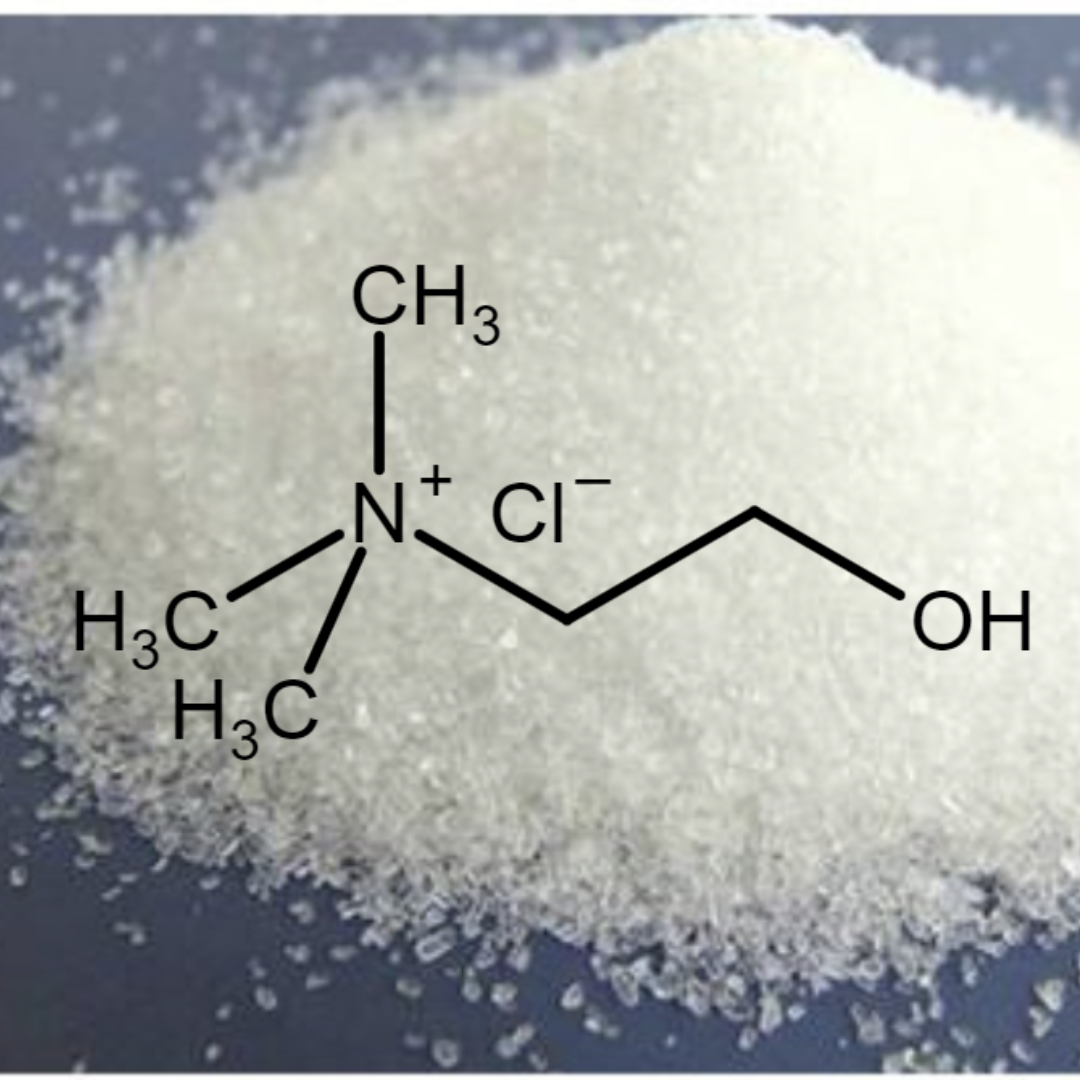
Choline chloride (ChCl)
Choline chloride (ChCl) is a vital quaternary ammonium salt, an essential nutrient (often called vitamin B4) and precursor for cell membranes, used in animal feed, supplements, and as a clay stabilizer in oil/gas, known for its water solubility and roles in cell signaling and liver health. It's a white powder or crystal, with formula C₅H₁₄ClNO, important for neurotransmission and overall growth.
We deliver turn key plant, and material supply

Key Functions in Animal Feed
Choline chloride is a vital, water-soluble nutrient, often called Vitamin B4, used as a feed additive in livestock (poultry, swine, pets, fish) to promote healthy metabolism, prevent fatty liver syndrome, support bone development (preventing perosis), and improve growth and reproduction. It functions as a lipotropic agent, aiding fat transport, a methyl group donor, and a precursor to neurotransmitters, making it crucial for cellular structure, nerve function, and overall nutrient utilization in growing animals.
Key Functions in Animal Feed
Lipid Metabolism: Prevents fat accumulation in the liver (Fatty Liver Syndrome) and aids in fat digestion and absorption.
Bone Health: Prevents skeletal deformities like perosis (slipped tendon) in young birds.
Growth & Performance: Enhances growth rates, feed efficiency, and carcass quality.
Reproduction: Improves egg production in poultry.
Cellular Function: A building block for phospholipids (cell membranes) and a source for acetylcholine (neurotransmitter).
Chorline Chloride Synthesis Process
Choline chloride synthesis primarily involves reacting trimethylamine (TMA) with ethylene oxide (EO) and hydrochloric acid (HCl), or using 2-chloroethanol instead of EO/HCl, often in a continuous or batch process to create the quaternary ammonium salt [(CH₃)₃N⁺CH₂CH₂OH]Cl⁻, a vital animal feed additive, with key steps including reactant mixing, reaction under controlled temperature/pressure, purification (evaporation, crystallization, drying) to achieve high purity for commercial use.
Propcess description
Ethylene Oxide & HCl Method (Most Common Industrial)
Reaction: Trimethylamine reacts with ethylene oxide, followed by neutralization with hydrochloric acid.
(CH₃)₃N + C₂H₄O + HCl → [(CH₃)₃N⁺CH₂CH₂OH]Cl⁻.
Process: TMA and EO are reacted in a continuous reactor, forming an intermediate, then HCl is added, followed by evaporation to separate the solid choline chloride.
General Process Steps (Example: EO/HCl Route)
Reactant Preparation: Trimethylamine (gas or aqueous solution), ethylene oxide, and hydrochloric acid are prepared.
Reaction: Trimethylamine and ethylene oxide are fed into a reactor, often with water, under controlled temperatures (e.g., 30-50°C) and pressure.
Neutralization: Hydrochloric acid is added to adjust the pH and form the final salt.
Purification:
Evaporation: Water is removed from the solution.
Crystallization: Choline chloride crystals are formed.
Separation & Drying: Crystals are separated (e.g., centrifugation) and dried to remove residual moisture, often using cold and hot air.
Chorline Chloride
.png)

Key Characteristics & Uses
Chemical Nature: A salt with a choline cation and chloride anion; contains both a quaternary ammonium group and a hydroxyl group, making it bifunctional.
Nutritional Role: Essential for synthesizing phospholipids (cell membrane components) and as a methyl donor; supports neurological function and liver health.
Animal Feed: Widely added to pet and livestock feed to promote growth and development.
Industrial Applications: Used as a highly effective clay stabilizer in water-based drilling fluids, preventing shale swelling.
Emerging Uses: Explored as a biodegradable solvent in deep eutectic solvents (DESs) for green chemistry.
Physical Form: Typically a white to off-white powder or crystal, highly soluble in water.
EQUIPMENT SUPPLY
DOWNLOADS
Add paragraph text. Click “Edit Text” to update the font, size and more. To change and reuse text themes, go to Site Styles.
UREA TO AMMOUNIA
.png)
Add paragraph text. Click “Edit Text” to update the font, size and more. To change and reuse text themes, go to Site Styles.
WFF EVAPORATOR
.png)
Add paragraph text. Click “Edit Text” to update the font, size and more. To change and reuse text themes, go to Site Styles.
